The Centre of New Technologies invites to a seminar by
Prof. Vivek Malhotra
Centre for Genomic Regulation, Barcelona
Secretion of bulky collagens from endoplasmic reticulum in health and disease
Date: May, 24th (Friday), 2019 at 12 p.m.
Venue: Centre of New Technologies, Banacha 2C,
Lecture Hall 0142 (Ground floor)
Host: Prof. Agnieszka Chacińska
Abstract:
Secreted collagens compose 25% of our dry body weight and necessary for tissue organization, and skin and bone formation. But how are these bulky cargoes that are too big to fit into a conventional COPII vesicle exported from the ER? Our discovery of TANGO1 (Bard, Nature 2006; Saito, Cell 2009; Saito, Mol Biol Cell 2011; Santos, J Cell Biol 2016; Malhotra, Ann Rev Cell Dev Biol 2019), a ubiquitously expressed, ER-exit-site-resident, transmembrane protein has made the pathway of collagen secretion amenable to molecular analysis. TANGO1 acts as a scaffold to connect collagens in the lumen to COPII coats on the cytoplasmic side of ER. However, the growth of the collagen containing mega transport carrier is not simply by accretion of a larger COPII coated patch of ER membrane, but instead by rapid addition of premade small vesicles. This mode of transport carrier formation is fundamentally different from that used to produce small COPII vesicles. We have seen that TANGO1 rings the ER exit site and thus organizies a sub compartment within the ER (Nogueira, eLife 2014; Santos, eLife 2015; Raote, J Cell Biol 2017). We have now mapped all the components that work in concert along with the cargo to assemble TANGO1 into a ring (Raote et al.,elife 2018.). Mathematical modelling, biochemistry and super resolution microscopy based analyses of this process will be discussed. We suggest that export of bulky collagens is by direct connection of ER exit site to the Golgi via transient tunnels created by the function of TANGO1 (Raote and Malhotra. J Cell Biol. 2019) TANGO1 is genetically mutated in patients with collagenopathies and in diseases like cylindromas. An understanding of how TANGO1 functions in exporting right quantity and quality of collagens will help control human pathologies due to defects in collagen dependent assembly of basement membrane and extracellular matrix.
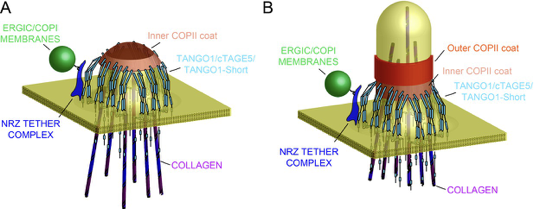
Model of TANGO1 ring assembly at an ERES.
(A) TANGO1-family proteins (cyan) assembly into a ring at an ERES is mediated by interactions 1. with COPII (orange) 2.with triple helical collagen (purple), 3. amongst the TANGO1 family proteins 4. with the NRZ tether (dark blue) which links TANGO1 to ERGIC membranes. TANGO1 delays the binding of the outer COPII coat to allow a mega carrier to form. (B) The cytoplasmic bud grows to a size that encapsulates collagen trimers. In this form, we suggest that the neck of this tubule is covered in the inner COPII coat bound to TANGO1, which prevents premature recruitment of outer COPII coat, thereby controlling the timing of membrane fission.


