
Mitochondria pełnią kluczową rolę w metabolizmie oraz procesach regulacyjnych komórki. Mitochondria są niezbędne do życia każdej komórki eukariotycznej począwszy od organizmów jednokomórkowych do ssaków.
W skład mitochondriów wchodzi od 1000 do 1500 białek komórkowych, które syntetyzowane są w cytozolu, poza mitochondrium.
Biogeneza mitochondriów jest zależna od wydajnego importu, sortowania i dojrzewania białek, za które odpowiedzialne są wysoce konserwatywne translokazy białkowe i inne złożone szlaki biologiczne. Nasze badania mają na celu powiązanie procesu transportu z procesami regulującymi homeostazę białek mitochondrialnych. Postawiona przez nas hipoteza badawcza zakłada istnienie mechanizmu wymiany informacji między cytozolem a mitochondrium, który zaangażowany jest w biogenezę białek.
Celem naszych badań jest dogłębne zrozumienie złożonych i dynamicznych procesów związanych z biogenezą mitochondriów jak również utrzymaniem homeostazy białkowej komórek oraz zaburzeniami, które skutkują patologiami.
Chętnie podejmiemy współpracę z wysoce zmotywowanymi i wykwalifikowanymi studentami i doktorantami. Zapraszamy do kontaktu: a.chacinska@cent.uw.edu.pl
Alumni:
dr Anna Antosiewicz
dr Arianna Barchesi
mgr Veronica Bazzani
dr Piotr Brągoszewski
mgr Żaneta Bugajska
dr Piotr Chrościcki
Aleksandra Gosk
dr Anna Kornakiewicz
mgr Łukasz Kowalski
mgr Monika Kwiatkowska
mgr Inés Juaristi Santos
mgr Tomasz Sitarz
mgr Vanessa Tolotto
dr Ulrike Topf
dr Barbara Uszczyńska-Ratajczak
dr Carlo Vascotto

Kuzniewska B., Cysewski D., Wasilewski M., Sakowska P., Milek J., Kulinski T.M., Winiarski M., Kozielewicz P., Knapska E., Dadlez M., Chacinska A., Dziembowski A., Dziembowska M.
EMBO Rep.
Kodroń A., Mussulini B.H., Pilecka I., Chacińska A.
Pharmacological Research
Mohanraj K., Nowicka U. & Chacińska A.
Biochem J (2020) 477 (16): 3033–3054
Pacheu-Grau, D., Wasilewski, M., Oeljeklaus, S., Gibhardt, C. S., Aich, A., Chudenkova, M., ... & Rehling, P.
Journal of molecular biology, 432(7), 2067-2079
Barchiesi, A., Bazzani, V., Tolotto, V., Elancheliyan, P., Wasilewski, M., Chacinska, A., & Vascotto, C.
Journal of Molecular Biology, 432(24), 166713
Turek, M., Piechota, M., Shanmugam, N., Niklewicz, M., Kowalski, K., Chacinska, A., & Pokrzywa, W.
bioRxiv
Chojnacka, K. J., Mohanraj, K., Callegari, S., Mussulini, B. H. M., Elanchelyan, P., Gosk, A., ... & Chacińska, A.
bioRxiv
Frankish, A., Diekhans, M., Ferreira, A., Johnson, R., ... & Uszczynska-Ratajczak, B. (2019).
Nucleic Acids Research, 47(D1), D766-D773
Topf U., Uszczynska-Ratajczak B., Chacinska A. (2019).
Journal of Cell Science, 132
Mohanraj, K., Wasilewski, M., Benincá, C., Cysewski, D., Poznanski, J., Sakowska, P., Bugajska, Z., Deckers, M., Dennerlein, S., Fernandez-Vizarra, E., Rehling, P., Dadlez, M., Zeviani, M. & Chacinska, A. (2019).
EMBO Mol Med, 11(5), pii: e9561.
Pereira, G. C., Allen, W. J., Watkins, D. W., Buddrus, L., Noone, D., Liu, X., Chacińska, A., . . . Collinson, I.
Journal of Molecular Biology, 431(8), 1689-1699
Monteuuis G, Miścicka A, Świrski M, Zenad L, Niemitalo O, Wrobel L, Alam J, Chacińska A, Kastaniotis AJ, Kufel J.
Nucleic Acids Res. 47(11):5777-5791
Samluk L, Urbanska M, Kisielewska K, Mohanraj K, Kim MJ, Machnicka K, Liszewska E, Jaworski J, Chacinska A.
Mol.Biol. Cell 30(15):1864-1877. doi: 10.1091/mbc.E18-10-0628.
Topf, U., Suppanz, I., Samluk, L., Wrobel, L., Böser, A., Sakowska, P., Knapp, B., Pietrzyk, M.K., Chacinska, A. and Warscheid, B. (2018).
Nature Communications, 9(1), p.324.
Kowalski L, Bragoszewski P, Khmelinskii A, Glow E, Knop M, Chacinska A. (2018).
BMC Biol. 2018 Jun 22;16(1):66. doi: 10.1186/s12915-018-0536-1.
Samluk, L., Chroscicki, P., & Chacinska, A. (2018).
Current Opinion in Physiology
Schendzielorz, A. B., Bragoszewski, P., Naumenko, N., Gomkale, R., Schulz, C., Guiard, B., ... & Rehling, P. (2018).
Nature communications, 9(1), 4028
Sokol, A. M., Uszczynska-Ratajczak, B., Collins, M. M., Bazala, M., Topf, U.,.... & Chacinska, A. (2018).
PLoS Genetics
Lewandowska, H., Stępkowski, T. M., Męczyńska-Wielgosz, S., Sikorska, K., Sadło, J., Dudek, J., & Kruszewski, M. (2018).
Journal of inorganic biochemistry, 188, 29-37
Uszczynska-Ratajczak, B., Lagarde, J., Frankish, A., Guigó, R., & Johnson, R.
Nature Reviews Genetics, 19(9), 535-548.
Bragoszewski, P., Turek, M., & Chacinska, A. (2017)
Open biology, 7(4), 170007
Gold, V.A., Chroscicki, P., Bragoszewski, P. and Chacinska, A. (2017)
EMBO reports, 18(10), pp.1786-1800.
| Tytuł | Termin nadsyłania aplikacji |
|---|---|
| PhD student in „ReMedy” International Research Agenda Unit | 08/12/2019 |
| Public Procurement and Financial Specialist | 30/07/2019 |
| Grant Office Specialist | 30/07/2019 |
| Postdoctoral researcher | 10/01/2019 |
| Bioinformatician / Computational Biologist | 16/12/2018 |
![]()
Project number: 2015/18/A/NZ1/00025
Project title: Cross-talk between the transport of mitochondrial proteins and cellular protein homeostasis
Project leader:
Agnieszka Chacinska, PhD, Professor
E: a.chacinska
Post-doctoral researchers:
Minji Kim, PhD
E: m.kim
Anna Antosiewicz, PhD
E: a.antosiewicz
PhD students:
Maria Śladowska, MSc
E: m.sladowska
Martyna Pietrzyk, MSc
E: m.pietrzyk
Source of funding: National Science Centre, Poland
Budget: 4 271 581 PLN
Project duration: 21.04.2016 – 20.04.2021
About the project:
Mitochondrion is a cellular compartment commonly known as “the power plant” of cells. To fulfill its various functions, this organelle needs more than one thousand cellular proteins. Yet, the majority of mitochondrial proteins are synthesized outside mitochondria in the cytosol and thus must be transported into mitochondria with the help of other proteins forming import machines. Dysfunctional mitochondrial protein import machines cause mitochondrial malfunctions, but also accumulation of precursor proteins in the cytosol. The Chacinska group discovered the role of the cytosolic degradation machinery in precursors’ clearance and the mechanism called the unfolded protein response activated by mistargeted proteins (UPRam) that protects the cell from stress caused by mistargeted mitochondrial precursor proteins accumulating in the cytosol. These processes pinpoint an important crosstalk between the state of mitochondria and regulatory mechanisms responsible for maintaining the cellular protein homeostasis. In the Maestro project, using simple model organisms, such as yeasts and worms, in addition to cultured mammalian cells, multidisciplinary approaches based on biochemistry, molecular cell biology and systems biology will be undertaken to identify and characterize the mechanisms of both, degradation of mistargeted mitochondrial proteins and the UPRam. We also aim to uncover biological consequences of these mechanisms, which are critical for homeostasis, survival and ageing at the cellular and organismal level.
Publications:
Wasilewski M., Chojnacka K., Chacinska A. (2017) Protein trafficking at the crossroads to mitochondria. Biochim Biophys Acta, 1864(1):125-137. https://doi.org/10.1016/j.bbamcr.2016.10.019
Lectures at the international conferences:
10.2018
Chacinska A.– Invited Speaker, CRC 1218 International Syposium: Mitochondrial Plasticity in Metabolism and Signalling, Max Planck Institute for Biologie of Ageing, “Guided Tour of proteins into mitochondria”, Cologne, Germany
07.2018
Chacinska A.– Invited Speaker, FASEB Conference, Protein Folding in the Cell, “Cellular protein homeostasis responses driven by mitochondria”, Buffalo, USA
Chacinska A. – Invited Speaker, The 43rd FEBS Congress, Theodor Bücher Plenary Lecture, „Mitochondria in cellular protein homeostasis”, Prague, Czech Republic
Pietrzyk M., et al. – The 43rd FEBS Congress, Poster: „Ribosome – associated quality control (RQC) in Saccharomyces cerevisiae upon oxidative stress”.
Śladowska M., et al. – Mitochondrial and Chloroplast Gordon Research Conference, Poster: „Mitochondrial protein import stress can prolong life of Caenorhabditis elegans„.
06.2018
Chacinska A. – Invited Speaker, Cell Symposia: Multifaceted Mitochondria, “The guided tour of proteins to mitochondria”, San Diego, USA
05.2018
Chacinska A. – Invited Speaker, EMBO Workshop: Molecular Biology of Mitochondria, “Mitochondria and cellular protein homeostasis mechanisms”, Stockholm, Sweden
09.2017
Chacinska A. – Invited Speaker, FEBS Congress: Protein Trafficking at the Crossroads to Mitochondria, Jerusalem, Israel
07.2017
Chacinska A. – Invited Speaker, EMBO Workshop: Mitochondrial quality control, “UPS-dependent degradation of mitochondrial precursor”, Xi’an, China
11.2016
Chacinska A. – Invited Speaker, International Conference of the Centre for Misfolding
Diseases 2016, “Protein homeostasis at the crossroads to mitochondria”,
Sevilla, Spain
11.2016
Chacinska A. – Invited Speaker, Protein Biogenesis & Mitochondrial Dynamics, “Protein
trafficking at the crossroads to mitochondria”, Baiersbronn-Obertal, Germany
09.2016
Turek M., et al. – The 2nd Congress BIO 2016: “Expanding beyond the limits”, Wroclaw, Poland
Posters at the international conferences:
09.2016
Śladowska M., Topf U., Chacinska A., Poster Session at the 7th EMBO meeting, “Effects of mitochondrial dysfunction in Caenorhabditis elegans”, Mannheim, Germany
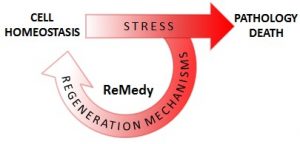 |
The „Regenerative Mechanisms for Health” International Research Agenda Unit (“ReMedy”) is a joint unit of the University of Warsaw and University Medical Center Göttingen at Georg-August-University Göttingen, funded by a grant by the Foundation for Polish Science. The goal of ReMedy is to understand and to harness stress‐evoked adaptability of cells at the molecular and biochemical level, in order to combat human diseases and pathologies. |
| Director: prof. Agnieszka Chacińska |
| Deputy director: prof. Magda Konarska |
| Project coordinator: dr Michał Wrzesiński |
| Administration Manager: mgr Marzena Niedźwiadek |
| Project and Lab Managers: dr Agnieszka Gajewska, dr Iwona Pilecka |
| Financial Administrative Specialist: mgr Sebastian Pomirski – Ciura |
ReMedy aims to:
|
| Prof. Chacińska will seek to identify global consequences of translational inhibition in human cells. Preliminary data show that although acute stress leads to rapid inhibition of protein synthesis, this stress is not lethal and frequently cells become even more resistant to further insults. Unknown adaptive mechanisms lead to re-initiation of protein translation. Prof. Chacińska’s group will undertake a system analysis of gene expression changes that accompany dysfunctional mitochondria, aiming to identify changes in transcription various steps of mRNA biogenesis and stability, including splicing – in collaboration with the Konarska lab – as well as translation activity/recruitment of mRNA during translation initiation. Studies of the group will deliver a comprehensive gene expression profiling under mitochondrial dysfunction and will lead to a discovery of yet unknown responses and adaptive pathways that have a potential to rescue cells and organisms from organellar stress and may in general benefit cellular and organismal fitness.
Prof. Konarska group will study the mechanisms by which aging or environmental signals influence the function of the splicing machinery and affect splicing outcomes. Changes in patterns of alternative splicing in higher eukaryotes are characteristic signatures of stress and disease, but little is known about the underlying mechanisms. These studies will help to understand how environmental changes or ageing affect regulation of gene expression at the splicing level. They will also help to understand the function of the spliceosome and suggest new ways to modulate it. Ultimately, the mechanisms utilized by yeast to regulate pre-mRNA splicing will also be validated in mammalian cells, and the way how they affect more complex regulation of alternative splicing will be studied in collaboration with the Chacińska group. |
 |
| ReMedy is funded by Foundation for Polish Science International Research Agendas Programme (project MAB/2017/2) under measure 4.3 „International Research Agendas“, Smart Growth Operational Programme 2014-2020. |
mgr Michał Bazała
mgr Veronica Bazzani
mgr Anita Brewińska
mgr Magdalena Chojnacka
dr Piotr Chrościcki
mgr Tomasz Czerwik
mgr Magdalena Długołęcka
mgr Jakub Dominowski
dr Beata Drabarek
mgr Aleksandra Fergin
mgr Edyta Głów
dr Agnieszka Górnicka
dr Elżbieta Januszewicz
mgr Inés Juaristi Santos
dr Magdalena Kaus-Drobek
dr Adrianna Łoniewska-Lwowska
mgr Aleksandra Matusiak
mgr Inmaculada Mora Espi
mgr Kamila Ornoch
mgr Sabine Poerschke
dr Paulina Sakowska
dr Anna Sokół
dr Małgorzata Sztolsztener
mgr Krzysztof Tarasiuk
mgr Vanessa Tolotto
mgr Agata Trojanowska
dr Aksana Varabyova
dr Lidia Wróbel


